Cultivated meat is expensive, but costs are dropping. The biggest hurdle? Growth media, which can cost hundreds of pounds per litre. To compete with traditional meat, prices must fall to £1 per litre or less. Three strategies are driving this change:
- Cell Line Engineering: Tweaking animal cells to reduce nutrient needs and grow efficiently.
- Media Optimisation: Replacing costly pharmaceutical-grade ingredients with cheaper food-grade alternatives.
- Bioreactor Improvements: Scaling production with larger, more efficient systems.
Each approach tackles a unique challenge, but their combined progress is key to making cultivated meat affordable. The goal? Bringing prices closer to conventional meat, making it accessible for everyone.
Engineering Cell Lines for Cultured Meat and Sustainable Cellular Agriculture #culturedmeat
1. Cell Line Engineering
Cell line engineering offers a clever way to cut nutrient costs by tweaking animal cells to produce their own growth factors. Instead of constantly adding expensive growth factors to the culture medium, scientists are enabling cells to create these nutrients themselves through autocrine signalling.
In 2024, Andrew J. Stout and his team at Tufts University successfully engineered bovine muscle cells to produce their own FGF2 [4][2]. Kevin Kayser, Chief Scientific Officer at Upside Foods, summed up the approach perfectly:
"Let's build a process and then select for a cell line that does what we want it to do. It's going to be a lot more up-front work, but in the end it's going to translate to better cost" [1].
Cost Reduction Potential
Recombinant proteins and growth factors are the biggest cost drivers in the production process [3]. By engineering cells to generate their own growth factors, companies can eliminate the need for costly external supplements - something that would otherwise require a near 99% cost reduction to make cultivated meat commercially viable [5]. Additionally, modifying cells to grow in suspension, rather than requiring a surface, enables the use of massive stirred-tank bioreactors (some exceeding 20,000 litres), significantly increasing production efficiency [2].
Implementation Timeframe
This approach isn’t without its challenges. Developing and characterising a new cell line typically takes 6 to 18 months [3], which contrasts sharply with the much shorter production cycle of just 2–8 weeks, from cell bank to harvest [3]. By 2023, almost half of cultivated meat companies were already exploring genetic engineering for either research or commercial purposes [3], positioning the industry to further refine cost-saving strategies.
Technical Challenges
There are still hurdles to overcome. Maintaining genomic stability and achieving immortalisation for indefinite cell proliferation - while ensuring cells can still differentiate properly - remains a tough nut to crack [4][3]. Additionally, the availability of cell lines with the right traits is still limited [4]. These challenges highlight the complexity of cell line engineering, but the potential rewards make it a promising avenue for reducing costs. Up next, we’ll explore media optimisation strategies.
2. Media and Growth Factor Optimisation
Reducing the costs of growth media is a key strategy in making cultivated meat more affordable. Right now, cell culture media is the biggest expense in cultivated meat production [5][3]. By cutting down these costs, there's a huge opportunity to bring prices closer to what consumers are willing to pay.
To hit a target price of £8 per kilogramme, media costs need to drop by over 99.9% from their current pharmaceutical-grade levels. Growth factors alone would need to be limited to just £0.80 per kilogramme [3][5]. As the Good Food Institute explains:
"The largest challenge the cultivated meat industry faces is not simply foregoing animal components in the cell culture media but rather discovering how to do so affordably and how to optimise affordable formulations for maximised productivity" [3].
Cost Reduction Potential
One of the main approaches to cutting costs is replacing pricey pharmaceutical-grade ingredients with cheaper, food-grade alternatives. For example, albumin, which accounts for 96.6% of recombinant protein needs, is being targeted with plant-based substitutes like rapeseed and chickpeas. Likewise, companies are swapping out individual amino acids for more affordable plant hydrolysates [5][3][1].
Progress is already being made. In August 2024, Believer Meats introduced an animal-component-free medium that costs just £0.50 per litre [6]. Using continuous manufacturing methods like tangential flow filtration, their analysis showed that cultivated chicken could be produced at £5 per pound in a 50,000-litre facility - making it competitive with organic chicken prices [6]. Similarly, Mosa Meat, in partnership with Nutreco, demonstrated that switching from pharmaceutical-grade to food-grade amino acids alone could cut costs by a factor of 100, all without sacrificing cell yield [1].
Implementation Timeframe
Compared to cell line engineering, media optimisation can deliver results much faster. While developing new cell lines can take 6 to 18 months [3], reformulating media often leverages existing food-grade supply chains, speeding up the process. Susanne Wiegel, Head of Alternative Protein Programme at Nutreco, sums it up well:
"Feeding cells isn't that different from feeding animals. The majority of nutrients are provided through agricultural crops" [1].
Technical Challenges
Despite the promise of cost savings, using food-grade ingredients comes with challenges. These ingredients can introduce impurities, batch inconsistencies, and potential impacts on cell performance and product quality [5][2]. Additionally, scaling up production to meet demand is no small feat. Capturing just 1% of the global meat market would require millions of kilogrammes of recombinant albumin - far exceeding current production levels for many industrial enzymes [5].
Next, we’ll explore how improvements in bioreactors and processes can further drive down costs.
sbb-itb-c323ed3
3. Bioreactor and Process Improvements
After refining cell line engineering and optimising media, the next step in reducing costs for cultivated meat lies in improving bioreactors and production processes. While cell lines and media focus on the biological side, the physical systems - bioreactors and manufacturing workflows - play a crucial role in making cultivated meat more affordable. As the Good Food Institute aptly states:
"The bioprocess design holds the key to unlocking large-scale production of cultivated meat" [3].
Right now, most bioreactors used in cultivated meat production are adapted from the pharmaceutical industry. These systems are designed for high-value, low-volume outputs, which isn’t ideal for the cost-efficient, high-volume demands of food production [3]. To compete with traditional meat, the industry needs purpose-built bioreactors designed specifically for large-scale, economical production. This is where process optimisations can help drive costs down further.
Cost Reduction Potential
One of the most promising ways to cut costs is by moving from pharmaceutical-grade to food-grade production standards. Unlike pharmaceutical applications, cultivated meat only needs to meet food safety standards, which are less stringent. This shift could significantly reduce operational expenses [3].
Process efficiency is another critical factor. Techniques like recycling growth media, repurposing waste streams, and implementing automation can help minimise the use of costly inputs [3]. For example, in September 2023, Upside Foods announced its plans for a commercial-scale facility near Chicago. This facility aims to produce 13,000 tonnes of cultivated meat annually using bioreactors as large as 100,000 litres [1]. Kevin Kayser, the company’s Chief Scientific Officer, highlighted the importance of focusing on raw material inputs:
"One of the reasons I was hired was raw material inputs... When I first started, it was top of the list" [1].
Scalability
Scaling up bioreactors is essential to achieve price parity with conventional meat. Currently, pilot-scale facilities use bioreactors ranging from 100 to 1,000 litres. However, techno-economic analyses suggest that achieving competitive pricing will require bioreactors with volumes of 20,000 litres or more - possibly even 100,000 litres [3][1][2]. By the end of 2024, at least one company had successfully scaled up to bioreactors with a 15,000-litre capacity [3].
The industry is moving through distinct phases: from bench-scale research (bioreactors under 10 litres), to pilot-scale testing, and eventually to industrial-scale production. Each stage demands not only larger equipment but also innovations in mixing, oxygen delivery, and monitoring systems [3].
Technical Challenges
Scaling up bioreactors isn’t without its challenges. Larger bioreactors bring unique technical difficulties, such as managing shear forces during mixing and oxygenation, which can damage delicate cells [3]. Oxygen transfer becomes increasingly complex as bioreactor volumes grow, and maintaining sterility in large-scale, food-grade facilities is critical - any contamination could result in significant production losses [3].
As Kevin Kayser noted, the industry is exploring new territory:
"When you start talking about 100,000 L or greater, I don't know if that's going to take any change in the media. We haven't gotten to that level" [1].
Unlike media optimisation, which can leverage existing food supply chains, scaling bioreactors requires solving entirely new engineering problems, especially at these unprecedented sizes [3].
Implementation Timeframe
Building industrial-scale facilities is a time-intensive and capital-heavy process. While developing a new cell line might take 6 to 18 months [3], constructing and commissioning a full-scale production facility takes years of planning and substantial investment [3]. However, new technologies are helping to speed things up. For instance, automated and cloud-powered systems have been shown to reduce development cycles by 25% and improve scale-up success rates by 30% [7]. Chris Williams, CEO of Culture Biosciences, explained:
"The shift toward cloud-powered, modular bioprocessing is accelerating in the biotech and biopharma sectors... It offers a flexible, cost-effective solution for teams that require faster development cycles and scalability" [7].
The cultivation process itself - from cell banking to harvest - typically takes 2 to 8 weeks, depending on the type of meat being produced [3]. Advances in bioprocessing will be critical to making cultivated meat a competitive option in the market.
Comparing the Three Approaches
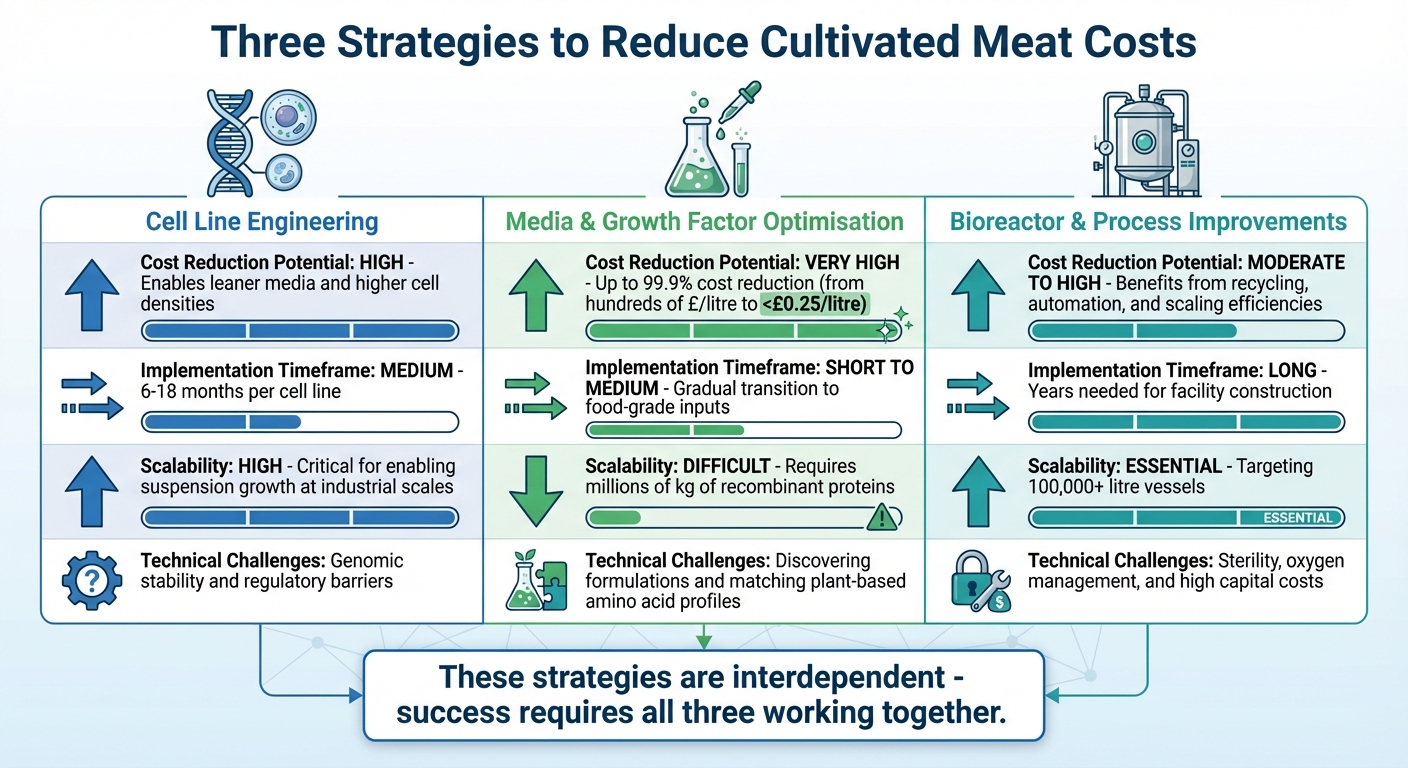
Comparing Three Strategies to Reduce Cultivated Meat Production Costs
Looking at cell line engineering, media optimisation, and bioreactor advancements side by side reveals how interconnected these strategies are. Each brings its own strengths and hurdles to the table, but together, they create a pathway to reducing costs in cultivated meat production.
Here’s a breakdown of how these approaches stack up across four key criteria:
| Criterion | Cell Line Engineering | Media & Growth Factor Optimisation | Bioreactor & Process Improvements |
|---|---|---|---|
| Cost Reduction Potential | High – allows for leaner media and higher cell densities | Very High – could slash costs by up to 99.9% from current biomedical prices | Moderate to High – benefits from recycling, automation, and scaling efficiencies |
| Implementation Timeframe | Medium – usually takes 6–18 months per cell line | Short to Medium – involves gradual transition to food-grade inputs | Long – years needed for facility construction and commissioning |
| Scalability | High – critical for enabling suspension growth at industrial scales | Difficult – requires production of millions of kilogrammes of recombinant proteins | Essential – targeting 100,000-litre+ vessels for large-scale production |
| Technical Challenges | Genomic stability and regulatory barriers | Discovering formulations and matching plant-based amino acid profiles | Ensuring sterility, oxygen management, and handling high capital costs |
Each strategy plays a distinct role in tackling the cost challenges of cultivated meat.
Media optimisation stands out for its immediate potential to slash costs. Prices could drop from hundreds of pounds per litre to less than £0.25 per litre [3]. However, scaling this approach to meet industrial demands is a significant obstacle.
Cell line engineering, on the other hand, lays the groundwork for success. By enabling suspension growth and reducing media requirements, it supports both media optimisation and bioreactor scaling [3]. Without reliable cell lines, progress in the other areas would stall.
Bioreactor improvements are a long-term game. Developing and commissioning facilities capable of handling 100,000-litre vessels is a daunting task, but it’s essential for scaling up to commodity-level production [3]. The engineering challenges here, particularly around sterility and oxygen transfer, remain largely uncharted territory at this scale.
The reality is that no single approach can carry the weight of cost reduction alone. These strategies are deeply interdependent. For instance, affordable media only holds value if bioreactors can operate at high volumes, and large-scale bioreactors only make sense if the media they use is cost-effective [3]. Together, these efforts create a cohesive framework that’s critical for making commercial-scale cultivated meat a reality.
Conclusion
Cell line engineering plays a key role in driving the success of both media refinement and bioreactor advancements. By developing cells that grow faster, reach higher densities, and perform well in leaner media, it significantly cuts costs tied to nutrients and bioreactor capacity. This makes it a cornerstone in reducing production expenses.
Media refinement offers immediate savings, with the potential to cut media costs by as much as 99.9%, bringing pharmaceutical-grade prices down to less than £0.20 per litre [3]. However, these savings hinge on cell lines that can thrive on such cost-effective media. At the same time, advanced bioreactor designs pave the way for large-scale production, but their economic viability depends on pairing them with affordable media and resilient, engineered cell lines.
The timeline for achieving price parity with premium conventional meat in the UK will be shaped by how quickly these three strategies - cell engineering, media development, and bioreactor scaling - advance together. Progress in these areas will form the foundation for making cultivated meat more accessible.
For UK consumers, widespread availability will also depend on regulatory approval, which is still under review as of late 2025 [3], and the creation of large-scale production facilities. Companies are already planning 100,000-litre bioreactors and aiming for facilities capable of producing up to 13,000 tonnes annually [1], signalling that the necessary infrastructure is taking shape.
The journey to affordable cultivated meat will hinge on the seamless integration of these three strategies. A production ecosystem combining engineered cells, low-cost media, and industrial-scale bioreactors will determine when cultivated meat moves from niche dining experiences to everyday supermarket offerings.
FAQs
How does cell line technology help lower the cost of cultivated meat?
Advances in cell line technology have brought down the cost of producing cultivated meat by improving the performance of the cells used in the process. These specially designed cell lines grow quickly, can thrive in dense environments, and withstand tough conditions such as low oxygen levels and mechanical stress. This means less reliance on costly growth media and more efficient, high-yield production in bioreactors.
By cutting down feedstock and processing costs, cell line technology is helping to make cultivated meat more accessible. This progress is a step toward establishing it as a practical alternative to traditional meat.
What are the main challenges in using food-grade media for cultivated meat production?
Switching to food-grade media for producing cultivated meat comes with some tough challenges. One of the biggest obstacles is cost. Right now, growth-factor-rich media - essential for cell growth - makes up more than half of production expenses. To bring costs down, manufacturers need to shift from expensive pharmaceutical-grade ingredients to cheaper, food-compatible alternatives. But here’s the catch: developing these affordable options, whether through precision fermentation or plant-based methods, is still in its early days and requires a lot of investment.
Another major issue lies in meeting strict food safety standards. Food-grade media must be produced under sterile conditions, free from any contaminants, and meet European Union food regulations. This adds layers of complexity to supply chains and quality control processes. On top of that, removing serum - commonly used in research-grade media - creates new waste management challenges. Without serum acting as a natural buffer, by-product build-up becomes a problem, demanding advanced recycling or removal systems.
There’s also the issue of cell adaptation. Many cell lines, originally developed for serum-based environments, struggle to grow in chemically defined, animal-free media. This can lead to slower growth or weaker cells, often requiring genetic tweaks to the cell lines or the creation of specialised supplements. Tackling these challenges is critical to scaling up cultivated meat production and making it more affordable and accessible to consumers. If you’re curious to learn more about this fascinating field, the Cultivated Meat Shop offers plenty of resources to explore.
How do large-scale bioreactors help make cultivated meat more affordable?
Large-scale bioreactors, especially those with capacities exceeding 20,000 litres, play a key role in lowering the production costs of cultivated meat. These systems allow for the production of large quantities of meat, which helps spread out expenses like equipment, labour, and growth media over a greater output. This approach helps achieve economies of scale, pushing cultivated meat closer to matching the price of traditional meat.
With this level of production, manufacturers can dramatically cut the cost per kilogram, paving the way for cultivated meat to become a more affordable and viable option for consumers.













